
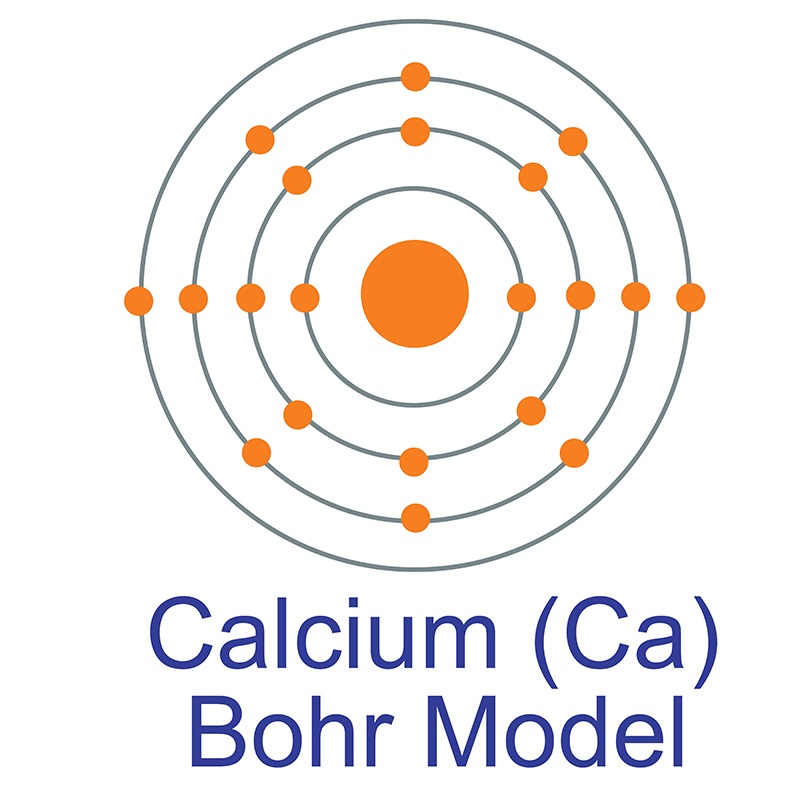
It does not store any personal data.\): The three most stable isotopes of hydrogen. The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. The cookie is used to store the user consent for the cookies in the category "Performance". This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. The cookies is used to store the user consent for the cookies in the category "Necessary". It is produced in the silicon-burning process from fusion of alpha particles and is the heaviest stable nuclide with equal proton and neutron numbers its occurrence is also supplemented slowly by the decay of primordial 40 K. This cookie is set by GDPR Cookie Consent plugin. By far the most common isotope of calcium in nature is 40 Ca, which makes up 96.941 of all natural calcium. The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". The cookie is used to store the user consent for the cookies in the category "Analytics". These cookies ensure basic functionalities and security features of the website, anonymously. Necessary cookies are absolutely essential for the website to function properly. You can do the reverse unit conversion from moles Calcium to grams, or enter other units to convert below:
#Charge of calcium 40 how to#
How to convert calcium to moles in units? Use this page to learn how to convert between grams Calcium and mole. Note that rounding errors may occur, so always check the results. The SI base unit for amount of substance is the mole. What is the formula for calcium in grams? When calculating molecular weight of a chemical compound, it tells us how many grams are in one mole of that substance. 1 mole is equal to 1 moles Calcium Carbonate, or 100.0869 grams.įinding molar mass starts with units of grams per mole (g/mol). The molecular formula for Calcium Carbonate is CaCO3. Molecular weight of Calcium Carbonate or grams. Voltages and Specific Gravity are listed for a 6-volt or 12-volt battery, and battery banks of 24 and 48 volts. How many grams are in a mole of calcium carbonate? I put together the following battery state of charge chart which indicates the state-of-charge (percent) as it relates to battery voltage or specific gravity. The mass of calcium ions required to supply one mole of charge of calcium ions is the mass divided by the charge, (40/2=20). For example, the mass of one mole of calcium ions is 40 g. It is the atomic mass or molecular weight divided by the charge.

What is the mass of 1 mole of chalk?Ĭonvert the grams of chalk from #4 into MOLES of chalk (the molar mass of CaCO3 is 100.09 g/mole) _ Use dimensional analysis, and show your work! Hence, the mass of 0.2 moles of CaCo3 is 20 g. What is the mass of 0.2 moles of calcium carbonate? Based on that information, to convert 1.5 moles of calcium to grams, we multiply 1.5 moles of calcium by 40.08.Įxplanation: The mass of 1 mole of calcium is 40.1⋅g. That means that one mole of calcium weighs 40.08 grams (40.08 g/mol). What is the mass of 1.5 moles of calcium?įurthermore, the atomic mass of calcium is 40.08.

How many grams are in 1 mole of calcium carbonate?ģ90 Unit 6 Changes in Matter The formula mass of CaCO3 is 100.09 amu and, therefore, the molar mass of one mole of CaCO3 is 100.09 grams. What is the mass of 0.5 moles of calcium carbonate? How do you calculate the mass of one mole of calcium carbonate? Natural calcium is a mixture of five stable isotopes (40Ca, 42Ca, 43Ca, 44Ca. 2 What is the mass of 1.5 moles of calcium? charged Ca2+ cation compared to the hypothetical Ca+ cation.If a third charge, of 5.00 nC, is now placed at the point x 3.20 cm, y 4.10 cm find the x and y components of the total force exerted on this charge by the other two charges. 1 How do you calculate the mass of one mole of calcium carbonate? A charge of -3.05 nC is placed at the origin of an xy-coordinate system, and a charge of 2.45 nC is placed on the y axis at y 4.10 cm.


 0 kommentar(er)
0 kommentar(er)
